Above a certain content of manganese, nickel, or some other elements, the γ state exists as stable from room temperature to melting point. Such highly alloyed iron alloys are called austenitic steels. Unlike other iron alloys, austenitic steels (and ferritic) do not undergo transformations upon heating and cooling. Therefore, heat treatment to harden austenitic steels is not used.
Cold resistant austenitic steels also include chromium manganese steels (austenitic steels in which nickel is completely or partially replaced by manganese); stable austenitic chromium nickel manganese steels with nitrogen (austenitic steels simultaneously alloyed with chromium, nickel and manganese) and metastable austenitic steels.
Lit .:
Existing austenitic high alloy steels and alloys are distinguished by the content of the main alloying elements - chromium and nickel and the composition of the alloy base. Highly alloyed austenitic steels are considered iron-based alloys alloyed with various elements in an amount of up to 55%, in which the content of the main alloying elements - chromium and nickel is usually not higher than 15 and 7%, respectively. Austenitic alloys include iron-nickel alloys with an iron and nickel content of more than 65% with a nickel to iron ratio of 1: 1.5 and nickel alloys with a nickel content of at least 55%.
Austenitic steels and alloys are classified according to the alloying system, structural class, properties and official purpose. Highly alloyed steels and alloys are the most important materials widely used in chemical, oil, power engineering and other industries for the manufacture of structures operating in a wide temperature range. Due to the high mechanical properties at low temperatures, high alloy steels and alloys are used in some cases as cold-resistant. An appropriate selection of alloying elements determines the properties and main service purpose of these steels and alloys.
A characteristic feature of corrosion-resistant steels is a reduced carbon content (not more than 0.12%). With appropriate alloying and heat treatment, the steels have high corrosion resistance at 20 ° C and elevated temperature both in a gaseous medium and in aqueous solutions of acids, alkalis and in liquid metal environments.
Heat-resistant materials include steels and alloys with high mechanical properties at elevated temperatures and the ability to withstand loads when heated for a long time. To impart these properties, steels and alloys are alloyed with hardening elements - molybdenum and tungsten (up to 7% each). An important alloying additive introduced into some steels and alloys is boron, which contributes to grain refinement.
Heat-resistant steels and alloys are resistant to surface chemical destruction in gaseous media at temperatures up to 1100-1150 0 С. They are usually used for lightly loaded parts (heating elements, furnace fittings, gas piping systems, etc.). High scale resistance of these steels and alloys is achieved by alloying with aluminum (up to 2.5%) and silicon, which contribute to the creation of strong and dense oxides on the surface of parts that protect the metal from contact with the gas environment.
According to the alloying system, austenitic steels are divided into two main types: chromium-nickel and chromomanganese. There are also chromium-nickel-molybdenum and chromium-nickel-manganese steels.
Depending on the main structure obtained by cooling in air, the following classes of austenitic steels are distinguished: austenitic-martensitic, austenitic-ferritic, austenitic.
Alloys on iron-nickel (with a nickel content of more than 30%) and nickel bases are structurally stable-austenitic and do not have structural transformations upon cooling in air.
At present, austenitic-boride Kh15N15M2BR1 (EP380), Kh25N20S2P1 (EP532), KhN77SR1 (EP615) and high-chromium austenitic KhN35VYu (EP568), KhN50 (EP668) steel and alloys with basic and non-ferrous alloys are also used. respectively.
After appropriate heat treatment, high alloy steels and alloys have high strength and plastic properties. In contrast to carbon, these steels acquire hardened plastic properties upon hardening. The structures of high alloy steels are diverse and depend not only on their composition, but also on the heat treatment regimes, the degree of plastic deformation, and other factors.
In austenitic chromium-nickel steels alloyed with titanium and niobium, not only chromium carbides are formed, but also titanium and niobium carbides. With a titanium content of Ti\u003e (% C-0.02) x5] or niobium Nb\u003e (% Cx10) all free carbon (above its solubility limit in austenite) can be released in the form of titanium or niobium carbides, and the austenitic steel becomes not prone to intergranular corrosion. The precipitation of carbides increases the strength and lowers the plastic properties of steels. This property of carbides is used for carbide hardening of heat-resistant steels, carried out in combination with intermetallic hardening by particles. Intermetallic compounds also include the α phase, which is formed in chromium-nickel steels during prolonged heating or slow cooling at temperatures below 900–950 ° C. It has limited solubility in α- and γ-solid solutions and, precipitating mainly along grain boundaries, hardens alloy and at the same time sharply reduces the plastic properties and toughness of the metal. Elevated concentrations in chromium steel (16–25%) and ferritizing elements (molybdenum, silicon, etc.) contribute to the formation of the σ phase at 700–850 ° C. This phase is released predominantly with the formation of an intermediate ferrite phase (γ → α → σ) or δ-ferrite transformations (δ → σ).
However, it is possible to isolate it directly from the solid solution (γ → σ).
In chromium-manganese steels with a high content of chromium and manganese, delayed cooling also results in the release of the σ phase. Carbon in chromium-manganese and chromium-manganese-nickel steels leads to precipitation hardening of steels after appropriate heat treatment, especially when combined with carbide-forming elements (vanadium, niobium and tungsten).
Hardening of austenitic-boride steels occurs mainly due to the formation of borides of iron, chromium, niobium, carbon, molybdenum and tungsten. In accordance with these processes, austenitic steels are subdivided, depending on the type of hardening, into carbide, boride, and intermetallic hardening. However, in most cases, due to the content in steels and alloys of a large number of different alloying elements, their hardening occurs due to the complex effect of dispersed phases and intermetallic inclusions.
Welding Features
The main difficulties in welding the steels and alloys under consideration are due to multicomponent alloying and the variety of operating conditions of welded structures. The main and general feature of welding is the tendency to form hot cracks in the seam and heat-affected zone, which have an intergranular character. They can be observed both in the form of the smallest micro-bursts and visible cracks. Hot cracks can occur during heat treatment or construction at elevated temperatures. The formation of hot cracks is associated with the formation of a coarse-grained macrostructure during welding, which is especially pronounced in multilayer joints, when the crystals of the next layer continue the crystals of the previous layer, and the presence of shrinkage stresses.
Metal welds Cellular dendritic crystallization forms are characteristic, which leads to the formation of large columnar crystals and enrichment of the interdendritic regions with impurities that form low-melting phases. In austenitic joints, the columnar structure is most pronounced. The use of methods that contribute to the grinding of crystals and the elimination of the columnar structure increases the resistance of the joints against the formation of hot cracks. One of these methods is to obtain joints with a certain amount of primary δ-ferrite in the structure. The positive effect of ferrite in austenitic-ferritic joints on the prevention of the formation of hot cracks in them is associated with a change in the crystallization pattern and a greater solubility of liquor impurities in it. The simultaneous precipitation of austenite and primary δ-ferrite crystals from the liquid phase leads to a refinement and disorientation of the structure, i.e., to a decrease in the cross-section of columnar crystals separated by sections of primary δ-ferrite. As a result, the probability of the formation of hot cracks at the locations of the liquid interlayers decreases. Obtaining austenitic-ferritic joints is achieved by their additional alloying with ferrite-forming elements, such as chromium, silicon, aluminum, molybdenum, etc. In products operating as corrosion-resistant at temperatures up to 400 0 C, ferrite content up to 20-25% is allowed. In products from heat-resistant and heat-resistant steels operating at higher temperatures, in order to prevent sigmatization, the amount of δ-ferrite in the joints is limited to 4-5%.
In steels with a large margin of austeniticity, it is difficult to obtain joints with an austenitic-ferritic structure. The ability to prevent hot cracks in them is achieved by limiting the content in the seams of impurities that form fusible eutectics (phosphorus, sulfur). To do this, use welding materials made of vacuum smelting steel or electroslag remelting, and limit the penetration of the base metal. In some cases, it is possible to improve the resistance of welds against hot cracks by increasing the content of liquor impurities to concentrations that ensure that, at the final stages of crystallization, abundant eutectics are obtained on the surface of crystallites, for example, when steel is alloyed with boron (0.3-1.5%). In this case, deformations accumulated in the weld metal toward the end of crystallization are reduced due to a decrease in the upper temperature of the effective crystallization interval. Reducing the effect of the force factor (current limitation, filling the grooves with rollers of a small cross section, rational design of the joint, etc.) is also a factor in preventing hot cracks.
In addition to the difficulty of producing welded joints without hot cracks on austenitic high alloy steels and alloys, there are other welding features due to the peculiarities of their use. Welded joints of heat-resistant steels are required to maintain high mechanical properties at elevated temperatures for a long time. High cooling rates during welding lead to the fixation of nonequilibrium structures in the weld metal. During operation at temperatures above 350 0 C as a result of diffusion processes, new structural components appear in the steel, leading to a decrease in the plastic properties of the weld metal. Thermal aging at 350-500 0 С causes the appearance of "475-degree brittleness", and at 500-650 0 С it leads to precipitation of carbides and at the same time to the formation of the α-phase. Exposure at 700-850 0 C intensifies the formation of the α phase with the corresponding strong embrittlement of the metal at lower temperatures and a decrease in strength at high temperatures. At the same time, the role of intermetallic hardening also increases. The processes of carbide and intermetallic hardening take the leading place in the processes of thermal aging of austenitic steels; therefore, to reduce the tendency of welded joints of heat-resistant and heat-resistant steels to embrittlement as a result of carbide precipitation, it is effective to reduce the carbon content in the base metal and weld metal.
In the heat-affected zone of some heat-resistant austenitic steels, the plastic and strength properties decrease under the action of the thermal welding cycle, which can lead to the formation of cracks in this zone. Such changes in the properties of the base metal are caused by the development of diffusion processes leading to an increased concentration of surface-active elements (carbon, oxygen, etc.) in the metal of the heat-affected zone, which together with other impurities can form fusible eutectics and ultimately cause the appearance of hot cracks. In addition, during prolonged use, finely dispersed carbides and intermetallic compounds can be released in this zone. The formation of a continuous layer of carbides and intermetallic compounds along grain boundaries leads to embrittlement of the weld. When welding these steels to prevent hot cracks in the weld, a deposited metal is often obtained, which differs in composition from the main one and has a two-phase structure. However, during high-temperature operation, carbide and intermetallic hardening of such a deposited metal and a corresponding decrease in its plastic properties occur, which leads to localization in the heat-affected zone of deformations and the formation of cracks in it. Significant residuals contribute to this. welding voltageas well as operating voltages. Prevention of such local fractures is achieved by heat treatment: austenization at 1050-1100 0 С to relieve residual welding stresses, self-hardening and impart more uniform properties to the welded joint. In some cases, austenization is accompanied by subsequent stabilizing annealing at 750-800 0 С to obtain relatively stable structures as a result of precipitation of the carbide and intermetallic phases. Local fractures are characteristic of the area of \u200b\u200boverheating of the heat-affected zone and are intercrystalline fractures due to the concentration of deformations along grain boundaries and the development of processes of intergranular slip. The hardening of grain boundaries of steel of type X16H9M2 due to molybdenum, which forms carbides at the grain boundaries, as well as a decrease in carbon content (up to 0.02%) or an increase in boron content up to 0.5% in steels 1X15H24V4T and 1X14N14V2M, respectively, increase the steel resistance to local fracture. Another way to reduce the tendency to local damage is to obtain a more ductile weld metal.
When welding high strength steels in the heat-affected zone, the formation of cold cracks is possible. Therefore, it is recommended that they be austenitized prior to welding to obtain high plastic properties of the metal, and hardening heat treatment should be carried out after welding. Preliminary and concurrent heating to 350-450 0 С also reduces the risk of the formation of cold cracks.
When welding heat-resistant steels under the influence of heating in the weld metal, the same structural changes can be observed as when welding heat-resistant steels. Most heat-resistant steels and alloys have a large margin of austeniticity and therefore do not undergo phase transformations during welding, except for carbide and intermetallic dispersion hardening. The formation of cold cracks in the weld and heat-affected zone is also possible on these steels, the prevention of which in some cases can be achieved by preheating up to 2 50-550 0 С.
Highly alloyed austenitic steels and alloys are most often used as corrosion-resistant. The main requirement for welded joints is resistance to various types of corrosion. Intergranular corrosion can develop both in the weld metal and in the base metal at the fusion lines (knife corrosion) or at some distance from the weld. The mechanism of development of these types of corrosion is the same, however, the causes of these types of intergranular corrosion are different.
Intergranular corrosion in the weld metal occurs as a result of precipitation from austenite under the influence of the thermal cycle of welding of chromium carbides, leading to depletion of boundary grain volumes by chromium. The main reasons for this are the increased content of carbon in the weld metal and the absence or insufficient content of titanium or niobium. The resistance of the weld against intergranular corrosion decreases as a result of prolonged exposure to heat during an unfavorable thermal cycle of welding or operation of the product. Austenitic-ferritic joints with a continuous structure and curved grain boundaries have an increased resistance to intergranular corrosion compared to austenitic ones. An increase in the length of grain boundaries due to grain refinement increases the surface area on which carbides are released. The precipitated carbides are more dispersed, and local depletion of the grain volume by chromium occurs to a lesser depth. In addition, diffusion processes in ferrite occur much faster, which accelerates the equalization of chromium concentration in depleted border and central parts of grains.
Intergranular corrosion (MCC) of the base metal at some distance from the weld is also caused by the action of the thermal welding cycle on that part of the base metal that has been heated to critical temperatures.
The tendency of steel and welds to intergranular corrosion is prevented:
1) a decrease in the carbon content to the extent of its solubility in austenite (to 0.02-0.03%);
2) alloying with more energetic than chromium carbide-forming elements (stabilization by titanium, niobium, tantalum, vanadium, etc.);
3) stabilizing annealing at 850-900 0 С for 2-3 hours or austenization - quenching from 1050-1100 0 С;
4) the creation of an austenitic-ferritic structure with a ferrite content of up to 20-25% by additional alloying with chromium, silicon, molybdenum, aluminum, etc. However, such a high content in the structure of ferrite can reduce the resistance of the metal to general corrosion.
The same measures contribute to the prevention of knife corrosion.
Knife corrosion affects the base metal. This type of corrosion develops in steels stabilized by titanium and niobium in areas heated during welding to temperatures above 1250 0 C, where titanium and niobium carbides dissolve in austenite. Repeated thermal exposure of this metal to critical temperatures of 500-800 0 С (for example, during multilayer welding) will lead to the conservation of titanium and niobium in the solid solution and the precipitation of chromium carbides.
General corrosion, i.e., dissolution of the metal in a corrosive environment, can develop in the weld metal, on various sites or in the heat-affected zone as a whole and in the base metal. In some cases, uniform general corrosion of the base metal and the welded joint is observed.
There is another type of corrosion failure - corrosion cracking that occurs under the combined action of tensile stresses and aggressive environment. Destruction develops both intergranular and transcrystalline. Reducing residual welding stresses is one of the main measures to combat this type of corrosion damage.
General welding conditions
Austenitic steels and alloys have a set of positive properties, so the same steel can sometimes be used for the manufacture of products for various purposes: corrosion-resistant, cold-resistant or heat-resistant. Moreover, the requirements for the properties of welded joints and welding technology will be different. However, the thermophysical properties of austenitic steels and the tendency to form hot cracks in the weld and heat-affected zone determine some common features of their welding.
Typical for most high alloy steels, low thermal conductivity and high linear expansion coefficient under the same heat input and other conditions being the same (welding method, edge geometry, joint rigidity, etc.) expand the penetration zone and regions heated to different temperatures and increase the total plastic deformation of the weld metal and the anterior zone. This increases the warpage of products. Therefore, for high alloy steels, welding methods and modes should be used, characterized by a maximum concentration of thermal energy, or reduce the current compared to the current when welding carbon steel. Heating to a high temperature of the welding wire in the overhang or the metal rod of the electrode for manual welding due to the increased electrical resistivity in automatic and semi-automatic arc welding requires a decrease in the stick-out of the electrode to increase its feed rate. With manual arc welding, the length of the electrodes and the permissible density of the welding current are reduced.
When welding austenitic steels, plastic deformation of the weld metal and the heat-affected zone as a result of high linear expansion and shrinkage coefficients, as well as the absence of polymorphic transformations, occurs to a greater extent than when welding pearlite-grade carbon steels (table 1). Under these conditions, in multilayer welding, the metal of the weld zone and the first layers of the weld metal can be hardened by repeated plastic deformation, i.e., the phenomenon of self-hardening is observed during welding. The effect of this phenomenon on the properties of the weld metal is determined by the stiffness of the elements being welded (table 2). In relatively more rigid joints, where self-hardening causes an increase in strength characteristics, an increase in residual stresses is observed in some cases up to 450-500 MPa. Such relatively high residual stresses with low relaxation ability of austenitic steels require the choice of a heat treatment mode that provides a reduction in residual stresses, removal of self-hardening and the maximum possible homogenization of the welded joint structure.
Among the main difficulties arising in the welding of austenitic steels is the need to increase the resistance of weld metal and heat-affected zone to cracking. Hot cracks are intergranular fracture and are divided into crystallization and subsolidus; the latter occur at a temperature below the solidus line, i.e., after the end of the crystallization process. The likelihood of crystallization cracks is determined by the nature of the change in the ductility of the alloys upon deformation of the metal in a solid-liquid state.
Table 1. Thermophysical properties of chromium-nickel austenitic steels
Table 2. Properties of the weld metal made by austenitic electrodes CT-7
The following ways of increasing resistance to the formation of crystallization cracks are proposed:
1) suppression of columnar crystallization and grinding of the crystal structure by alloying with modifier elements, as well as elements that contribute to the formation of high-temperature second phases during crystallization;
2) an increase in the purity of alloys by impurities, contributing to the formation of crystalline fusible phases in the range of compositions in which an increase in the number of these phases reduces the technological strength, and, conversely, an increase in the number of alloying elements forming eutectics in the composition of alloys close to eutectic. These paths narrow the temperature range of brittleness and increase the ductility margin.
Technological measures to combat cracks are aimed at finding rational methods and modes of fusion welding and structural forms of welded joints that reduce the rate of increase of internal deformations during the solidification process. Intergranular fracture of single-phase austenitic welds at temperatures below the solidification temperature under growing stresses (subsolidus cracks) according to the scheme is close to fracture during high-temperature creep. Intergranular slippage, which reveals both steps at the boundaries and already existing microcavities, formed as a result of vacancies at the boundaries perpendicular to the action of tensile stresses, is a necessary condition for the formation of embryonic cracks of such a fracture.
To increase the resistance of metals and their single-phase alloys, the formation of sub-solidus hot cracks during welding is recommended:
1) alloying alloys with elements that reduce the diffusion mobility of atoms in the lattice or contribute to the creation of a fragmentary cast structure (curvature of crystallite boundaries, the formation of dispersed second phases and precipitates during crystallization during subsequent cooling);
2) increasing the purity of the base metal by the introduction of impurities;
3) reducing the residence time of the metal at a temperature of high diffusion mobility (increasing the cooling rate of the weld metal) and reducing the rate of increase of elastic-plastic deformations during cooling (limiting deformations by choosing a rational design of joints).
The following most important metallurgical factors have been identified that contribute to increasing the resistance of the weld metal to the formation of hot cracks during welding of austenitic steels:
1) the formation of a two-phase structure in the high-temperature region during crystallization of the metal due to the separation of primary ferrite, dispersed particles of the refractory phase or boride phase and chromium-nickel eutectic;
2) limiting the content of impurities that form low-melting phases in order to narrow the effective crystallization interval.
To grind the structure, alloying the deposited metal with elements promoting the release of high-temperature δ-ferrite during crystallization of the metal is used. The presence of δ-ferrite grinds the metal structure and reduces the concentration of Si, P, S and some other impurities in intercrystalline regions due to the greater solubility of these impurities in δ-ferrite, which reduces the risk of the formation of low-melting eutectics. The amount of ferrite phase in the deposited metal after its cooling depends on the composition of this metal and the cooling rate in the region of high and medium temperatures. An approximate idea of \u200b\u200bthe concentration of ferrite in an austenitic-ferritic metal is given by the Scheffler diagram, compiled from experimental data as applied to the cooling rate, typical for ordinary manual arc welding (picture 1).
Figure 1. Scheffler diagram
The recommended content of the ferrite phase in the deposited metal is limited to 2-6%. When welding steels with a higher degree of austeniticity, for example, 08Kh18N12T, Kh14N14, etc., the limits of the content of the ferritic phase in the deposited metal are increased in order to ensure its presence in the weld taking into account mixing of the deposited metal with the base metal.
With an increase in the proportion of the base metal, for example, CT-15-1 (08X20H9G2) electrodes are used, which provide a structure containing 5.5-9% ferrite, or CT-16-1 (08X20H9BB) electrodes that provide a structure containing 6.0 -9.5% ferrite. Sometimes, when welding the root layers of multipass welds on steels of the 2Kh25N20S2 type, prone to the formation of crystallization cracks, GS-1 electrodes (10Kh25N9G6S2) are used, which provide a structure containing 25-30% ferrite in the deposited metal.
For corrosion-resistant steels, an increase in the content of primary ferrite to 15-25% improves the characteristics due to the greater solubility of chromium in ferrite than in austenite, which prevents depletion of the boundary layers by chromium and maintains high resistance to intergranular corrosion. For heat-resistant and heat-resistant steels with a small margin of austeniticity and a nickel content of up to 15%, the prevention of hot cracks is achieved by obtaining an austenitic-ferritic structure with 3-5% ferrite. A large amount of ferrite can lead to significant high-temperature embrittlement of welds due to their sigmatization in the temperature range 450-850 0 С.
Obtaining the austenitic-ferritic structure of welds on deep austenitic steels containing more than 15% Ni will require increased alloying with ferrite-forming elements, which will lead to a decrease in the plastic properties of the weld and embrittlement due to the appearance of brittle eutectics, and sometimes the a phase. Therefore, in the joints they seek to obtain an austenitic structure with finely divided carbides and intermetallic compounds and alloy the joints with an increased amount of molybdenum, manganese and tungsten, which suppress the formation of hot cracks. It is also necessary to limit the content of harmful (sulfur, phosphorus) and liquor (lead, tin, bismuth) impurities, as well as gases - oxygen and hydrogen in the main and deposited metals. To do this, one should apply regimes that reduce the proportion of the base metal in the weld, and use steel and welding materials with a minimum content of these impurities. Therefore, for the manufacture of welding wires, it is desirable to use vacuum melting steel, after electroslag remelting or refining: the same applies to the base metal. The welding technique should ensure minimal saturation of the weld metal with gases. This contributes to the use for welding direct current reverse polarity. For manual welding with coated electrodes, a short arc should be maintained and welding should be carried out without transverse vibrations. When welding in shielding gases, to prevent air leakage, it is necessary to maintain a short overhang of the electrode and choose the optimal welding speed and consumption of shielding gases.
Highly alloyed steels contain aluminum, silicon, titanium, niobium, chromium as alloying additives, which have a greater affinity for oxygen than iron. In the presence of an oxidizing atmosphere in the weld zone, their significant burnout is possible, which can lead to a decrease in the content or to complete disappearance of the ferritic and carbide phases in the weld structure, especially in a metal with a small excess of ferritizers. Therefore, it is recommended to use low-silicon high-base fluxes (fluoride) and electrode coatings (calcium fluoride) for welding. Short arc welding and air suction prevention serve this purpose. Nitrogen, being a strong austenitizer, simultaneously contributes to the refinement of the structure by increasing the centers of crystallization in the form of refractory nitrides. Therefore, the nitriding of the weld metal increases their resistance to hot cracks. High-base fluxes and slags, refining the weld metal and sometimes modifying its structure, increase resistance to hot cracks. Mechanized welding methods, ensuring uniform penetration of the base metal along the seam length and the constancy of the thermal welding cycle, allow one to obtain more stable structures along the entire length of the welded joint.
An important measure to deal with hot cracks is the use of technological methods aimed at changing the shape of the weld pool and the direction of growth of austenite crystals, as well as reducing the force factor resulting from the thermal welding cycle, shrinkage deformations and the rigidity of fixing the welded edges (Figure 2). Under the action of tensile forces perpendicular to the direction of growth of columnar crystals, the probability of cracking increases. In mechanized methods of welding with thin electrode wires, transverse vibrations of the electrode, changing the crystallization pattern of the weld metal, reduce the tendency of the weld metal to hot cracks. Reducing the action of shrinkage deformations is achieved by limiting the welding current, filling the grooves with seams of small cross-section, and using grooves of the corresponding structures. Good closure of the crater when the arc breaks contributes to this.

Figure 2. The influence of the welding coefficient on the technological strength of the weld metal type HYUN65M23
In addition to the general features listed above, welding of high alloy steels and alloys, there are features determined by their official purpose. When welding heat-resistant and heat-resistant steels, the required properties in many cases are ensured by heat treatment (austenization) at 1050-1100 0 С, which removes residual welding stresses, followed by stabilizing tempering at 750-800 0 С. If heat treatment is impossible, welding is sometimes performed with preliminary or concomitant heating to 350-400 0 C. Excessive embrittlement of the joints due to the formation of carbides is prevented by a decrease in the content of carbon in the joint. Providing the necessary heat resistance is achieved by obtaining a weld metal identical in composition to the base metal. This is also required to obtain joints that are resistant to general liquid corrosion.
When welding corrosion-resistant steels in various ways to prevent intergranular corrosion, one should not allow increasing the carbon in the weld metal due to contamination of the welding materials (graphite lubrication of the wire, etc.) and prolonged and repeated stay of the weld metal in the critical temperature range. Therefore, welding must be performed at the lowest heat input, using mechanized methods that ensure the continuity of the weld. Repeated arc excitation during manual welding, having an undesirable thermal effect on the metal, can cause its tendency to corrosion. A seam facing an aggressive environment should, if possible, be welded last, in order to prevent re-heating, and subsequent seams in multilayer seams should be made after the previous ones have completely cooled and measures should be taken to accelerate cooling of the seams. Sprays falling on the surface of the base metal can subsequently become foci of corrosion and must be carefully removed from the surface of the metal, welds, as well as residues of slag and flux, which, interacting with metal during operation, can lead to corrosion or a decrease in local heat resistance. During welding, the creation of an austenitic-ferritic structure in the weld metal to increase the resistance of welds to intergranular corrosion is achieved by alloying with titanium or niobium. However, titanium, which has a high affinity for oxygen, burns out in the welding zone by 70-90% (for manual arc welding, welding under acid fluxes). Therefore, alloying of welds with titanium is possible when welding in inert shielding gases, in arc and electroslag welding using fluoride fluxes. The content of titanium in the weld metal must correspond to a ratio Ti / C ≥ 5. Niobium oxidizes significantly less during welding and is therefore often used for alloying the weld in manual arc welding. Its content in the weld metal should correspond to a ratio of Nb / C\u003e 10. However, it can cause hot cracks to appear in the welds.
Manual arc welding
The main feature of welding austenitic steels is to provide the required chemical composition of the weld metal for various types of welded joints and spatial provisions welding, taking into account changes in the penetration depth of the base metal and the amount of deposited metal. This makes it necessary to adjust the composition of the coating in order to ensure the necessary content of ferrite in the joint and to prevent, thus, the formation of hot cracks in the joint, as well as to achieve the necessary heat resistance and corrosion resistance of the joints. The use of electrodes with a calcium fluoride (main) coating and the maintenance of a short arc without transverse vibrations of the electrode contribute to the production of a weld metal with the necessary chemical composition and structures and to reduce the burning of alloying elements. The latter also reduces the probability of formation of defects on the surface of the base metal as a result of adhesion of the spray.
The type of electrode coating determines the need to use a direct current of reverse polarity, the value of which is assigned so that its ratio to the diameter of the electrode does not exceed 25-30 A / mm. In the ceiling and vertical positions, the welding current is reduced by 10-30% compared with the current selected for the lower welding position.
Welding with coated electrodes is recommended to be performed with filament seams and to increase resistance to hot cracks, use electrodes with a diameter of 3 mm. In all cases, a minimum penetration of the base metal should be ensured. Before welding, the electrodes should be calcined at 250-400 0 C for 1-1.5 hours to reduce the likelihood of formation of pores caused by hydrogen and cracks in the welds.
The type of electrodes for welding high alloy steels with special properties is determined by GOST 10052-75. Dimensions and general technical requirements are regulated by GOST 9466-75.
Submerged arc welding
Submerged arc welding is one of the main processes for welding high alloy steels with a thickness of 3-50 mm in the production of chemical and petrochemical equipment. The main advantage of this method over manual arc welding with coated electrodes is the stability of the composition and properties of the metal along the entire length of the weld when welding with and without cutting edges. This is ensured by the possibility of obtaining a weld of any length without craters formed when changing electrodes, uniform melting of the electrode wire and the base metal along the length of the weld, and more reliable protection of the welding zone from oxidation of alloying components with atmospheric oxygen. Good formation of the surface of the welds with small scale and smooth transition to the base metal, the absence of splashes on the surface of the product significantly increase the corrosion resistance of welded joints. The complexity of preparatory work is reduced, since the cutting of edges is performed on metal with a thickness of more than 12 mm (for manual welding, on metal with a thickness of 3-5 mm). Welding is possible with an increased gap and without cutting the edges of steel up to 30-40 mm thick. Reducing waste losses, spatter and cinder of electrodes by 10-20% reduces the consumption of expensive welding wire.
The technique and modes of welding high alloy steels and alloys have a number of features compared to the welding of ordinary low alloy steels. To prevent overheating of the metal and the associated enlargement of the structure, the possibility of cracks and reduce the operational properties of the welded joint, it is recommended to weld with small cross-sections. This leads to the use of welding wires with a diameter of 2-3 mm, and taking into account the high electrical resistance of austenitic steels, the need to reduce the electrode outburst by 1.5-2 times. The austenitic welding wires during the manufacturing process are very sticky and have high rigidity, which complicates the work of the correct, feed and current-supply nodes of welding plants, reducing their service life.
The seam is alloyed through a flux or wire. The latter method is more preferable, as it provides increased stability of the composition of the weld metal. For submerged arc welding of austenitic steels and alloys use welding wires produced in accordance with GOST 2246-70 and departmental technical conditions, and low-silicon fluoride and highly basic fluoride-free fluxes that create non-oxidizing or low-oxidizing environments in the welding zone, contributing to a minimum waste of alloying elements. In fluxes used for corrosion-resistant steels, it is necessary to control carbon, the content of which should not be higher than 0.1-0.2%. Low-silicon fluxes AN-26, 48-OF-Yu and ANF-14 are most widely used for welding corrosive steels.
Heat-resistant steels are welded with 08Kh25N13BTYu-type austenitic-ferritic wires under the low-silicon fluxes AN-26, ANF-14 and 48-OF-10. When welding with stable austenitic wires and wires containing easily oxidizable elements (aluminum, titanium, boron, etc.), neutral fluoride fluxes ANF-5, 48-OF-Yu are used. To ensure resistance to hot cracks in austenitic joints, it is recommended to use fluoride boron flux ANF-22.
Welding under fluoride fluxes is carried out with direct current of reverse polarity, and under highly basic fluoride-free fluxes with direct current of direct polarity. At the same time, to obtain the same penetration depth as on carbon steels, the welding current should be reduced by 10-30%. To reduce the likelihood of pore formation in welds, fluxes for high alloy steels must be calcined immediately before welding at 500-900 0 C for 1-2 hours. Remnants of slag and flux on the weld surface must be carefully removed.
Submerged arc welding in combination with high alloy wires provides the required properties of welded joints.
Electroslag welding
The reduced sensitivity to the formation of hot cracks, which allows obtaining austenitic joints without cracks, is explained by the features of electroslag welding: the low speed of the heat source, the nature of the crystallization of the metal of the weld pool and the absence of butt joints large angular deformations. However, a long stay of the metal at 1200-1250 0 С, leading to irreversible changes in its structure, reduces the strength and plastic properties of the heat-affected zone, which increases the tendency of welded joints of heat-resistant steels to local (heat-affected) fractures during heat treatment or operation at elevated temperatures. When welding corrosion-resistant steels, overheating of the steel in the heat-affected zone can cause knife corrosion, so heat treatment of welded products (hardening or stabilizing annealing) should be performed.
For electroslag welding of corrosion-resistant steels, use fluxes ANF-6, ANF-7, ANF-8, 48-OF-6, ANF-14 and others, and for heat-resistant steels use fluxes ANF-Sh, ANF-7, ANF-8 and highly basic AN-292. When welding heat-resistant steels with a two-phase weld of the X25H13 type, low-silicon fluxes ANF-14 and AN-26 can be used. The use of non-oxidizing fluoride fluxes, especially when welding heat-resistant steels and alloys, does not guarantee the burning of easily oxidizing alloying elements (titanium; manganese, etc.) as a result of the penetration of air oxygen through the surface of the slag bath; this makes it necessary in some cases to protect the surface of the slag bath by blowing it with argon.
Electroslag welding can be performed with a wire with a diameter of 3 mm or plate electrodes with a thickness of 6-20 mm. Products of large thickness with seams of small length are more appropriate to weld with a plate electrode. It is simpler to produce a plate electrode than a wire, but wire welding makes it possible to change the shape of the metal bath and the nature of the crystallization of the weld, which contributes to welds without hot cracks. However, the rigidity of the welding wire complicates the long and reliable operation of the current-supplying and feeding nodes of the welding equipment.
Shielded gas welding
Inert gases (argon, helium) and active gases (carbon dioxide, nitrogen) are used as protective gases, as well as various mixtures of inert or active gases and inert gases with active ones.
Shielded gas welding can be used to join materials of various thicknesses (from tenths to tens of millimeters). The use of shielding gases with various thermophysical properties and their mixtures changes the thermal efficiency of the arc and the conditions for introducing heat into the welded edges and expands the technological capabilities of the welding process. When welding in inert gases, the stability of the arc increases and the fumes of alloying elements decrease, which is important when welding high-alloy steels. The specified chemical composition of the weld metal can be obtained by changing the composition of the welding (filler) wire and the participation of the base metal in the formation of the weld, when the compositions of the base and electrode metals differ significantly, or by changing the nature of metallurgical interactions due to a significant change in the composition of the protective atmosphere when welding with a consumable electrode . Welding in a shielding gas environment provides the formation of welds in various spatial positions, which allows this method to be used instead of manual arc welding with coated electrodes.
Welding of austenitic steels in inert gases is performed by a non-consumable (tungsten) or consumable electrode.
Welding with a tungsten electrode is carried out in argon according to GOST 10157 and helium or their mixtures and is usually used for material up to 5-7 mm thick. However, in some cases, such as welding fixed joints pipes, they are used with a large wall thickness (up to 100 mm or more). It is also necessary to apply this method for welding root joints in cutting during the manufacture of critical thick-walled products.
Depending on the thickness and design of the welded joint, welding with a tungsten electrode is carried out with or without filler material. The process is carried out manually using special burners or automatically with direct current of direct polarity. The exception is steel and alloys with a high aluminum content, when alternating current should be used to destroy the surface film of oxides rich in aluminum.
Welding can be performed continuously by a burning or pulsed arc. The pulse arc reduces the length of the heat-affected zone and warpage of the welded edges, and also ensures good formation of a seam on a material of small thickness. Features of the crystallization of metals in the weld pool with this welding method contribute to disorientation of the structure, which reduces the likelihood of hot cracks, but can contribute to the formation of heat-affected tears. To improve the protection and formation of the weld root, gas injection is used, and when welding root welds on metal of increased thicknesses, special molten inserts are also used. When welding with a tungsten electrode in inert gases by a submerged arc, an increase in the fraction of heat going to the molten base metal allows, without cutting edges, to weld metal of increased thickness in one pass. However, the heat-affected zone expands, and there is a danger of overheating of the metal.
High alloy steels are plasma welded. The advantages of this method are the extremely low consumption of shielding gas, the possibility of obtaining plasma jets of various sections (round, rectangular, etc.) and changing the distance from the plasma torch to the product. Plasma welding can be used both for sheet materials and metal up to 12 mm thick. Its use for joining steels of greater thickness is hindered by the possibility of formation of undercuts in the joints.
Welding with a consumable electrode is carried out in inert as well as active gases or a mixture of gases. When welding high-alloy steels containing easily oxidizable elements (aluminum, titanium, etc.), inert gases, mainly argon, should be used and the process should be carried out at current densities that ensure jet transport of electrode metal. During jet transfer, the arc has high stability, and metal spatter is virtually eliminated, which is important for the formation of welds in various spatial positions and to eliminate the corrosion centers associated with spatter during welding of corrosion-resistant and heat-resistant steels. However, jet transfer is possible at currents higher than critical, at which burnout may occur during welding of sheet metal. The addition of argon to 3-5% O 2 and 15-20% CO 2 reduces the critical current, while the creation of an oxidizing atmosphere in the arc zone reduces the likelihood of pore formation caused by hydrogen. However, when welding in the indicated gas mixtures, the fumes of the alloying elements increase, and when carbon dioxide is added, it is possible to carburize the weld metal. By adding 5-10% N to argon, its content in the weld metal can be increased. Nitrogen is a strong austenitizer, and thus the structure of the weld metal can be changed. For welding austenitic steels, pulsed-arc welding with a consumable electrode in argon and mixtures of argon with oxygen and carbon dioxide is used, which provides a connection of small thicknesses and jet transfer of metal during the passage of a current pulse. At the same time, pulsed-arc welding causes grinding of the weld structure and a decrease in overheating of the heat-affected zone, which increases the resistance of the welded joint against cracking.
When welding in carbon dioxide low carbon high alloy steels using low carbon welding wires, with the initial carbon concentration in the wire less than 0.07%, the carbon content in the weld metal rises to 0.08-0.12%. This is enough to sharply reduce the resistance of weld metal to intergranular corrosion. However, carburization of the weld metal in some cases with vigorous carbide formers (titanium, niobium) can have a beneficial effect in welding heat-resistant steels due to an increase in the amount of carbide phase in the structure.
The oxidizing atmosphere created in the arc due to the dissociation of carbon dioxide causes an increased (up to 50%) burnup of titanium and aluminum. Manganese, silicon and other alloying elements burn out somewhat less, and chromium does not oxidize. Therefore, when welding corrosion-resistant steels in carbon dioxide, welding wires containing deoxidizing and carbide-forming elements (aluminum, titanium and niobium) are used. Another disadvantage of welding in carbon dioxide is a large spray of metal (losses reach 10-12%) and the formation on the surface of the weld of dense films of oxides firmly adhered to the metal. This can dramatically reduce the corrosion resistance and heat resistance of the welded joint. To reduce the possibility of spray buildup on the base metal, special emulsions should be applied on the edges before welding, and to combat an oxide film, a small amount of ANF-5 fluoride flux can be fed into the arc. The use of pulsed welding also allows you to slightly reduce spatter. Welding with a consumable electrode in carbon dioxide is carried out on semiautomatic devices and automatic machines.
Welding wires designed for welding high-alloy austenitic steels in carbon dioxide provide the required corrosion resistance and mechanical properties due to the increased content of titanium, niobium and elements of ferritizers - silicon, aluminum, chromium. For example, for welding steels of type 12Kh18N10T, wires Sv-07Kh18N9TYu, Sv-08Kh20N9S2BTYu are used, for steels of type 12Kh18N12T, wire Sv-08Kh25N13BTY, and for chromium-nickel molybdenum steels, wires Sv-06Kh19N10MZT and SvZ 06T20N.
Shiny, non-corroding steel products are coated with chromium, molybdenum, tungsten and alloyed, the alloy of which contains the necessary additives to add strength, resistance to corrosion and temperature changes, such as:
- cobalt;
- aluminum;
- titanium;
- copper;
- manganese;
- nickel;
- chromium;
- vanadium;
- molybdenum;
- silicon.
Depending on the purpose of the steel, it may contain other substances that improve its technical characteristics and give it a luster and smooth surface.
The conformity of the stainless steel product is checked at a temperature equal to 20 ° C. The German Standardization Institute has created a system by which austenitic steels are divided into categories. A2 and A3 are the categories of chromium-nickel steels, A4 and A5 are the categories to which chromium nickel and molybdenum steel belong. The specific gravity of these steels is the same. Despite this, the load maintained by the steel object increases with an increase in the category number. The percentage of deformation increases with heating. Mechanical damage can occur only with a strong, directed impact force or with the use of special equipment - a press or a pipe bender.
In the cold state, steel is very resistant to stretching and other types of deformation. She has a high coefficient of resistance. When heated, this coefficient decreases by half, regardless of the category of steel, it is almost equal.
Considering that the melting temperature of austenitic steels occurs at a temperature of 1800 ° C, it is worth noting that its quenching occurs when heated to 850 ° C. Austenization occurs when heated above 1000 ° C. Its elasticity varies slightly with strong heating. The indicators are checked at temperatures of 300 °, 400 ° and 500 ° C.
When assembling metal fencing, creating composite metal products, 2 types of welding are used. Despite the fact that steel has good and excellent welding characteristics, it is necessary to understand the choice between arc and gas welding, because during the welding process the metal adjacent to the weld seam changes its structure, which affects the appearance and susceptibility of the metal. With continuous heating, the scale will appear at a temperature slightly above 900 ° C, with periodic heating, in order to avoid its manifestation, the heating must be reduced by 100 ° C.
Technology for welding austenitic steels
Stainless austenitic steel is melted at a temperature of almost 2000 ° C. But, despite this, the low carbon content in its composition gives excellent weldability. The temperatures of the welding machines are not so high that scale will form during the welding process. There are no unpleasant odors when heating stainless steel. To avoid warpage and intergranular corrosion, fast welding methods are used.
An improperly selected welding process and cooling mode can lead to undesirable consequences. During welding, not only the welding zone is heated, but also the adjacent metal sections. Their temperature can reach 700 ° C. At this temperature, chromium decomposes, which, upon slow cooling, will lead to the precipitation of its carbides. The austenitic structure of the steel at the carbide deposition sites will be disrupted, which will entail a decrease in all technical specifications and deplorably affect the appearance of the finished metal.
Oxidation of chromium may be accompanied by a refractory neoplasm. Most often, chromium oxide remains inside the seam. Its melting temperature is 100-200 ° C higher than that of stainless steel itself. Low thermal conductivity of steel with a high coefficient of linear expansion creates tension in the heat-affected zone. The low intensity of gas welding equipment, when metal heating occurs gradually, leads to the fact that the heating area increases. This contributes to a slight, slow cooling of the metal, causing precipitation of chromium oxidation products. When welding a hollow pipe, the oxidation products will appear inside it behind the weld (under the condition of free access of air into the pipe cavity).
The use of arc welding for a stainless steel is more appropriate, since in this process the seam is more even, the connection is reliable, and the steel retains its initial technical characteristics.
Gas welding is justified when fastening parts of small thickness, not exceeding 2 mm. The welding process is similar in temperature and flame intensity to that used for carbon steels. Welding filler material is a wire with the same composition as the stainless steel itself. If it contains titanium or niobium, then this will reduce the precipitation of chromium carbides.
Although they all belong to the class of high alloy steels. very good, pre-heating and subsequent heat treatment is not required. As a rule, they are not inclined to and, but this property applies to the steels themselves and does not apply to welds.
Austenitic steels contain 17% Cr and more. Such steels have much higher elongation, toughness and parameters of transition to a brittle state. In the annealed condition, they have a high yield index and, if necessary, these steels can be strengthened with deformation without fear of embrittlement.
Major grades and chemical composition of austenitic steels for welding
The main grades of welded austenitic steels, according to Russian standards, include: 12X17, 15X6SYU, 10X13SYU, 15X11MF, 15X25T, 08X18H10, 12X18H9, 12X18H9T, 08X18H10T, 12X21H5N, 17X17H17M2T, 20X25H20MNT2, 08X17X17H17M2T, 20X25H20H17M2T2, 08X17X17H13M2T2, 08X17X17M13T2. In addition to the above grades, there are also austenitic steels and alloys, but they are difficult because of their special properties.
The effect of chemical composition on weldability of austenitic steels
The main type of austenitic chromium-nickel steels is X18H10. The structure of such steels is austenitic, with some inclusion of delta ferrite (about 2-7%). With a nickel content of about 8%, austenite is partially converted to martensite at room temperature if the steel is subjected to plastic deformation.
Heat-resistant austenitic steels contain up to 25% chromium, and the nickel content can reach 38%. The heat resistance of steel is increased by alloying the steel with silicon (about 1%), or aluminum.
The metal structure of welds in austenitic steels is shown in the Scheffler diagram. The diagram shows the dependence of the metal structure on the equivalents of chromium and nickel. But, in addition to the elements indicated in the diagram, the percentage for copper with coefficients of 0.6 and nitrogen with a coefficient of 10-30 can be used in the expression for calculating the nickel equivalent. And in the formula for calculating the equivalent of chromium, the percentage coefficient of tungsten is 0.5 and titanium is 2-5.
The Scheffler diagram is usually used for conditions. When using other types of welding, the structure of the weld metal may differ from that shown in the diagram.
The main objective to ensure is to prevent the formation of cold and hot cracks. It was experimentally established that the propensity of the weld metal depends on the ferrite content in the steel. When the ferrite content is in the range of 2-6%, the risk of cracking is significantly reduced.
Researcher Delong refined the Scheffler diagram. But the content of the ferrite component changes significantly when taking into account the percentage of nitrogen with a coefficient of 30. This must be taken into account for (welding in shielding gases, welding with a consumable electrode and non-consumable). Therefore, the Delong diagram cannot also be considered absolute.

To evaluate the approximate ferrite content, Seferian derived the following expression: x \u003d 3 * (Cr-eq - 0.93Ni-eq - 6.7),%
The presence of the required amount of ferrite (2-6%) allows us to solve the problem of the absence of cracks in the welding of austenitic steels. But, at the same time, ferrite reduces the elongation of the weld metal, reduces the viscosity, increases the transition temperature and negatively affects the corrosion resistance.
In the deposited metal, in addition to microcracks, others can also form. And they are connected with the fact that sulfides and oxides present in the composition of steel cannot float to the surface of a liquid weld pool due to its high viscosity. Therefore, to reduce the viscosity of the molten metal, it is recommended to alloy steel with silicon in an amount of 0.3-0.7%.
Structural changes in the metal during welding of austenitic chromium steels
When welding austenitic steels in the heating zone, grain growth occurs. And it happens more smoothly than unalloyed structural steels. But, if there is an obstacle for this in the form of a carbide phase, then grain growth does not occur.
In the overheating zone, in addition to grain growth, the carbide phase dissolves, for the most part, it is Cr23 C6 carbide. In addition to chromium carbides, carbides of other stabilizing metals — titanium, niobium and vanadium — are also formed. In addition to Cr23 C6 carbides, chromium nitrides Cr2 N and Cr7 C3 carbides appear. The dissolution of part of the carbides leads to the formation of thin films of these carbides along the grain boundaries. Because of this, steel is highly susceptible to intergranular corrosion.
These transformations can be avoided by stabilizing the steel. But in the case of using such types of welding as electroslag welding, or submerged arc welding (high-performance), even stabilization does not solve the problem of intergranular corrosion.
You can increase the strength of the weld metal by adding a small amount of nitrogen.
Heating and heat treatment in welding austenitic steel
When welding austenitic steels, preheating, in terms of structural transformations, is not necessary. But, in some cases, apply heating to a temperature of 200 ° C in order to reduce internal stresses.
The value of residual stresses in such steels is quite large, because of this there is a risk of corrosion failure of the steel. In order to avoid this, heat treatment of welded joints is performed.
If it is only necessary to reduce the value of internal stresses, then the tempering temperature of 800-850 ° C is selected. If welded joints contact with the medium, which contributes to the formation of intergranular corrosion, it will be appropriate to perform annealing at a temperature of 950-1050 ° C. Annealing helps dissolve carbide films.
When performing heat treatment, it must be taken into account that steels of the type Х18Н8, Х18Н8М2, Х18Н8Т, Х18Н9Б, Х25Н12, Х25Н20 have a tendency to form tempering cracks.
Gas welding of austenitic steels
For austenitic steels, it is recommended to choose an acetylene-oxygen welding flame with a capacity of 70-75 l / h based on 1 mm of thickness to be welded. It is not recommended to use oxidative, as when it is used, chrome strongly burns out. austenitic steels are recommended for the following grades: Sv-02X19H9T, Sv-08H19N10B. Other grades of low carbon wire doped with titanium or niobium are also used. (1-6mm), the diameter of the wire is chosen equal to the diameter of the base metal.
Often used, for example, flux brand NZh-8. Flux components are mixed on liquid glass and applied to the welded edges of the product. The welding process is performed after the flux has completely dried.
Welding austenitic steels can be done by anyone, without limitation. The composition of filler materials is usually chosen similar to the composition of the welded steels. If the requirements for corrosion resistance are high, then the use of a filler material that does not contain a ferrite base will be appropriate.
Austenitic steels, having a number of special properties, are used in those working environments that are highly aggressive. Such alloys are indispensable in power engineering, at the enterprises of the oil and chemical industries.
1
Austenitic alloys include alloys with a high level of alloying, which upon crystallization usually form a single-phase system characterized by a crystal face-centered lattice. This type of lattice in the described steels remains unchanged even in those cases when the metal is cooled to very low temperatures, called cryogenic (in the region of -200 degrees Celsius). In some cases, austenitic-grade steels have another phase (its volume in the alloy can reach ten percent) - ferrite with a high degree of alloying. In this case, the lattice is body-centered.
The separation of austenitic steels into two groups is carried out by the composition of their base, as well as by the content in the alloy of alloying components - nickel and chromium:
- Compositions based on iron: nickel content - up to 7%, chromium - up to 15%, total amount of alloying additives - no more than 55%.
- Compositions on nickel (55% or more nickel) and iron-nickel base (they contain 65 and more percent of nickel and iron, with the ratio of the first to the second being 1 to 1.5).
In such alloys, nickel increases the ductility, heat resistance and processability of steel, and chromium is responsible for giving it the required corrosion and heat resistance. And by adding other alloying components, it is possible to achieve unique properties of austenitic compounds, a set of which determines the official mission of this or that alloy.
Most often, austenitic steels are alloyed with the following elements:
- Ferritors that stabilize the structure of austenite. These include vanadium, tungsten, niobium, titanium, silicon and molybdenum.
- Austenitizers, which are nitrogen, carbon and manganese.
All of these components are located both in excess phases and directly in solid steel solution.
According to the accepted classification, taking into account the alloying system, any austenitic steel can be classified as chromium-manganese or chromium-nickel. In addition, alloys are divided into chromium-nickel-manganese and chromium-nickel-molybdenum.
2
A variety of additives allows you to create special austenitic steels that are used to make parts for structures operating in high temperature, corrosive and cryogenic conditions. Based on this, austenitic compounds and are divided into different groups:
- corrosion resistant;
- cold resistant.
Heat-resistant compounds are not destroyed when exposed to a chemical environment. They can be used at temperatures up to +1150 degrees. A variety of lightly loaded products are made from such steels:
- elements of gas pipeline systems;
- fittings for furnace equipment;
- heating parts.

Heat-resistant steel grades can resist loads under high-temperature conditions for a long time, while maintaining their initially high mechanical characteristics. They are necessarily alloyed with tungsten and molybdenum (each of the additives can be contained in the steel composition in an amount of up to seven percent). And for grinding grain in some austenitic alloys, boron is introduced in small quantities.
We denote the common brand of heat-resistant and heat-resistant steels described in the article class: H15N35VTR, 10H12N20T3R, 40H18N25S2, 1H15N25M6A, 20X23H13, 10X15H18B4T, 10H16N14V2BR, 10X18H12T, 08H16N9M2, 10H15N35VT, 20H25N20S2, 1H15N25M6A, 20X23H13, 10X15H18B4T, 10H16N14V2BR, 10X18H12T.
Austenitic stainless steel (i.e., corrosion resistant) are characterized by a low carbon content (more than 0.12 percent of this chemical element is not allowed). Nickel in them can be from 8 to 30%, and chromium from 12 to 18%. Any austenitic stainless steel undergoes heat treatment (tempering, hardening or). Heat treatment is necessary so that stainless steel products feel good in different aggressive environments - in alkaline, gas, liquid metal, acid at temperatures from +20 degrees and more.

The following grades of austenitic corrosion-resistant steels are best known:
- chromium nickel-molybdenum: 03X21H21M4GB, 08X17H15M3T, 08X17H13M2T, 03X16H15M3, 10X17H13M3T;
- chromomanganese: 07X21G7AH5, 10X14AG15, 10X14G14H4T;
- nickel chromium: 08X18H12B, 03X18H11, 08X18H10T, 06X18H11, 12X18H10T, 08X18H10;
- with a high silicon content (from 3.8 to 6.7%): 15X18H12C4T10, 02X8H22C6.
Cold resistant austenitic compositions contain 8–25% nickel and 17–25% chromium. They are used for cryogenic devices, have a high production cost, therefore they are used very limited. The most common cryogenic steels are 07Kh13N4AG20 and 03Kh20N16AG6, which are alloyed with nitrogen. This element is introduced so that the alloy at a temperature of + 20 ° has a higher yield strength.
3
The most common are austenitic chromium-nickel steels that have molybdenum additives. They are used when there is a risk of formation of a crevice either. They demonstrate high resistance in reducing atmospheres, and are divided into two types:
- unstabilized by titanium with a carbon content of not more than 0.03%;
- stabilized by titanium with carbon from 0.08 to 0.1%.
Such grades of chromium-nickel compositions as X17H13M2 and X17H13M3 are optimal for structures operating in sulfuric acid media, in acetic ten percent acid, in boiling phosphoric acid.

Nickel-chromium steels with the addition of niobium or titanium are characterized by a minimal risk of intergranular corrosion. Compared to carbon, niobium is introduced 9–10 times more, and titanium 4–5.5 times more. The alloys with a similar possibility include the following compositions: 0X18H12B, 0X18H10T, X18H9T and some others.
It is also possible to increase the corrosion resistance of the described steels by introducing silicon into them. Bright representatives of such special compositions are such alloys:
- 015X14H19S6B;
- 03X8H22C6.

Without exaggeration, they are ideal for the production of chemical welded assemblies in which nitric concentrated acid is stored and processed.
Chromium-manganese steels of type 2X18H4GL are characterized by high casting characteristics, so they are used in industries where corrosion-resistant cast structures are used. Other chromomanganese alloys (for example, 10Kh13G12N2SA and 08Kh12G14N4YUM) in combustible media are more resistant to corrosion than chromium-nickel ones.
4
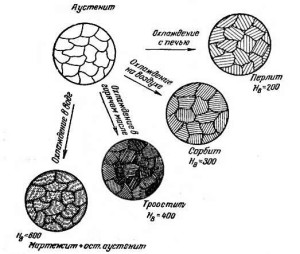
Heat-resistant and heat-resistant alloys of the austenitic group are subjected, if necessary, to various types of heat treatment in order to increase their properties, as well as to modify the existing grain structure: the number and distribution principle of dispersed phases, the size of the blocks and the grain itself, and so on.
Annealing of such steels is used to reduce the hardness of the alloys (when required by the conditions of their operation) and eliminate the phenomenon of brittleness. With this heat treatment, the metal is heated to 1200–1250 degrees for 30–150 minutes, and then it is cooled as quickly as possible. Complexes are most often cooled in oil or in air, but alloys with small amounts of alloying components are usually immersed in water.

For alloys of type ХН35ВТЮ and ХН70ВМТЮ, heat treatment in the form of double hardening is recommended. First, the first normalization of their composition is performed (at a temperature of about 1200 degrees), due to which the metal increases the creep resistance index due to the formation of a solid homogeneous phase. And after that, a second normalization is carried out with a temperature of not more than 1100 degrees. The result of the described treatment is a significant increase in the plastic and heat-resistant properties of austenitic steels.

Austenitic steel increases its heat resistance (and at the same time also mechanical strength) in those cases when double heat treatment takes place, consisting in hardening and aging following it. In addition, almost all austenitic metals, which belong to the heat-resistant group, artificially age before use (that is, they perform the operation of their dispersion hardening).


